Telomere Length vs. Epigenetic Age: Unraveling the Secrets of Cellular Aging
16 Aug 2023
0 comments
Telomeres and epigenetic age are both indicators of cellular aging, but they reflect different biological processes and have different implications. Let's explore each of these in detail:
-
Telomere Length:
- Definition: Telomeres are repetitive DNA sequences at the ends of chromosomes. They protect chromosomes from degradation, fusion, and other abnormalities.
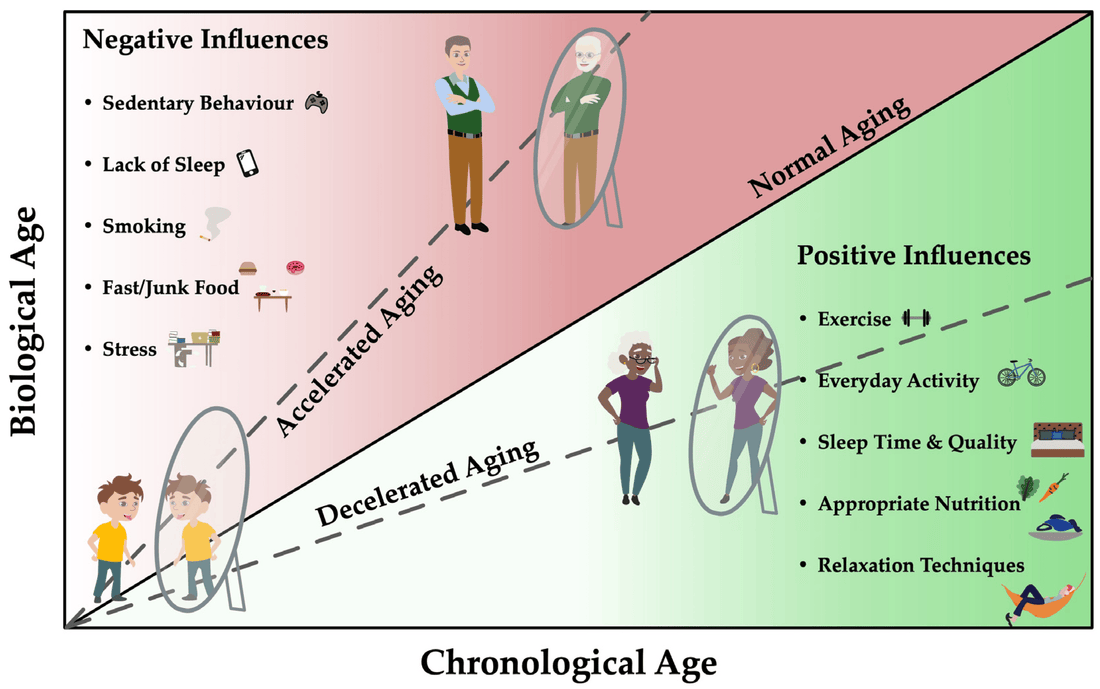
- Role in aging: Each time a cell divides, its telomeres shorten slightly. Over time, this shortening can accumulate and reach a critical point where the cell becomes senescent (stops dividing) or undergoes apoptosis (programmed cell death). Therefore, telomere length is often seen as a biological clock.
- Measurement: Telomere length is typically measured using techniques like qPCR, TRF analysis, or next-generation sequencing.
- Implications: Shortened telomeres are associated with increased disease risk and mortality, but this relationship is complex. Some research suggests that artificially lengthening telomeres might extend cellular lifespan but could come with risks like heightened cancer susceptibility.
-
Epigenetic Age:
- Definition: This refers to the age prediction based on the pattern of DNA methylation, a key epigenetic mark, across numerous specific genomic sites. In essence, it looks at chemical changes to the DNA molecule itself.
- Role in aging: DNA methylation patterns change as we age. Some sites become more methylated, while others become less so. The overall pattern of these changes can be used to estimate an individual's biological age – this is known as one's "epigenetic age."
- Measurement: DNA methylation can be measured using arrays or next-generation sequencing, and specific algorithms (like the Horvath Clock) can then estimate an individual's epigenetic age based on these patterns.
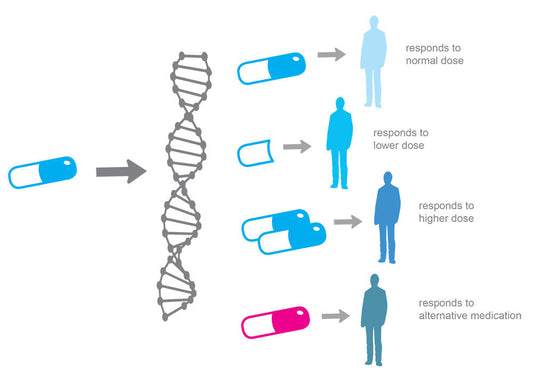
- Implications: The difference between epigenetic age and chronological age can have health implications. For instance, an "older" epigenetic age relative to one's actual age might indicate increased health risks. Epigenetic aging rates can be influenced by genetics, lifestyle, and environmental factors.
Comparison:
- Nature: Telomere length is a more direct measure of cellular aging and is based on physical attrition of chromosome ends. In contrast, epigenetic age is based on chemical modifications to the DNA molecule.
- Predictive Value: Both shortened telomeres and increased epigenetic age are associated with higher risks of various diseases and mortality. However, the predictive value can vary depending on the specific condition being studied.
- Interventions: There are interventions (like certain drugs and lifestyle changes) that might affect telomere attrition rates or even epigenetic aging rates. However, how to effectively "reverse" or "halt" aging in either of these metrics remains a topic of active research.
In summary, both telomere length and epigenetic age offer insights into the biological processes of aging, but they represent different mechanisms. It's also worth noting that aging is a multifaceted process, and these markers, while informative, are only pieces of a much larger puzzle.