A Guide to Toxins and Fillers: Treatment, Complications, and Management
Toxins and fillers have become increasingly popular in the cosmetic industry over the last few decades. These products are commonly used to improve the appearance of the face and reduce the signs of aging. In this article, we will discuss the history and pharmacology of toxins and fillers, patient assessment and administration of these products, treatment of specific facial areas, complication avoidance and management, on-label treatment of facial areas, and post-treatment management.
History and Pharmacology of Toxins and Fillers
Toxins were first used for cosmetic purposes in the 1970s when it was discovered that botulinum toxin type A (Botox) could be used to treat strabismus (crossed eyes). In the 1990s, Botox was approved by the FDA for cosmetic use in treating facial wrinkles. Fillers, on the other hand, have been used for over a century, starting with the use of paraffin in the late 1800s. However, due to the high rate of complications with paraffin, new materials were developed.
Fillers are categorized as either biodegradable or non-biodegradable. Biodegradable fillers are made from materials that can be broken down by the body, such as hyaluronic acid. Non-biodegradable fillers, such as silicone, are not broken down by the body and can cause long-term complications.
Toxins work by blocking the release of acetylcholine, a neurotransmitter that is responsible for muscle contraction. By blocking this neurotransmitter, muscles are relaxed, resulting in a smoother appearance of the skin. Fillers, on the other hand, work by adding volume to the face, filling in wrinkles and lines.
Patient Assessment and Administration of Toxins and Fillers
Before administering toxins or fillers, it is important to assess the patient's medical history, including any allergies or medical conditions they may have. It is also important to discuss the patient's expectations and goals for the treatment. Once the assessment is complete, the appropriate products can be chosen and administered.
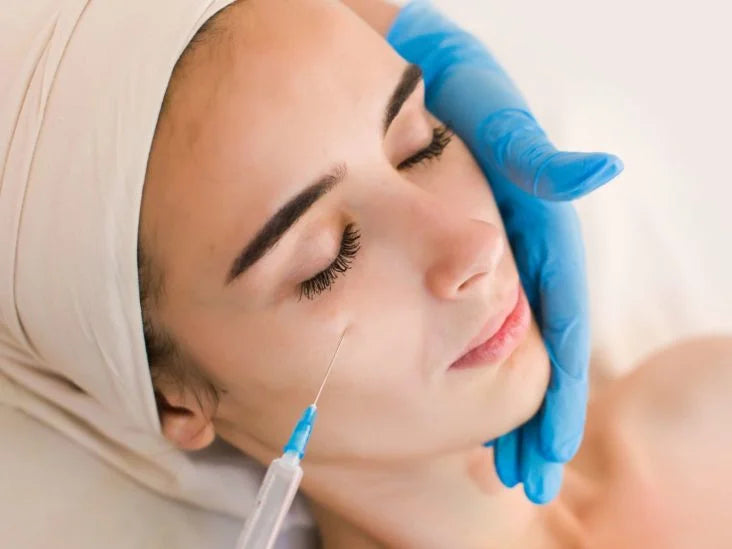
Toxins are typically administered via injection using a small needle. The injection sites will vary depending on the area being treated. Fillers can also be administered via injection or cannula, a small tube used to inject the product into the skin. The specific technique used will depend on the filler being used and the area being treated.
Treatment of Facial Areas
Toxins and fillers can be used to treat a variety of facial areas. Common areas treated with fillers include the cheeks, perioral areas, lips, nasolabial lines, and marionette lines. The use of toxins is primarily for treating dynamic wrinkles, such as frown lines and crow's feet.
When treating the cheeks, fillers can be used to add volume and contour the face. In the perioral area, fillers can be used to reduce the appearance of fine lines and wrinkles around the mouth. In the lips, fillers can be used to add volume and enhance the shape of the lips. Nasolabial lines and marionette lines can also be treated with fillers to reduce the appearance of wrinkles and fine lines.
Complication Avoidance and Management
Complications can occur with the use of toxins and fillers, such as bruising, swelling, infection, and allergic reactions. To avoid complications, it is important to choose the appropriate product for the patient and administer it correctly.
If complications do occur, they can be managed by administering medications to reduce swelling and inflammation, antibiotics to treat infections, or steroids to reduce allergic reactions. It is important to closely monitor the patient after the treatment to ensure any complications are managed appropriately.
On-Label Treatment of Facial Areas
The FDA has approved the use of toxins and fillers for specific facial areas. Botox is approved for use in the glabella (the area between the eyebrows), forehead lines, and crow's feet. Dysport and Xeomin are also approved for use in the glabella and forehead lines. Fillers, such as Juvederm and Restylane, are approved for use in the nasolabial folds, marionette lines, and lips.
It is important to follow the on-label guidelines when administering these products to ensure their safety and effectiveness.
Post-Treatment Management
After the treatment, patients should avoid rubbing or touching the treated area for several hours to reduce the risk of complications. Patients should also avoid strenuous exercise for 24-48 hours after the treatment to reduce swelling and bruising.
It is important to follow up with the patient after the treatment to assess their satisfaction and address any concerns they may have. The effects of toxins and fillers are not permanent and will eventually wear off. Patients will need to schedule follow-up appointments for additional treatments.